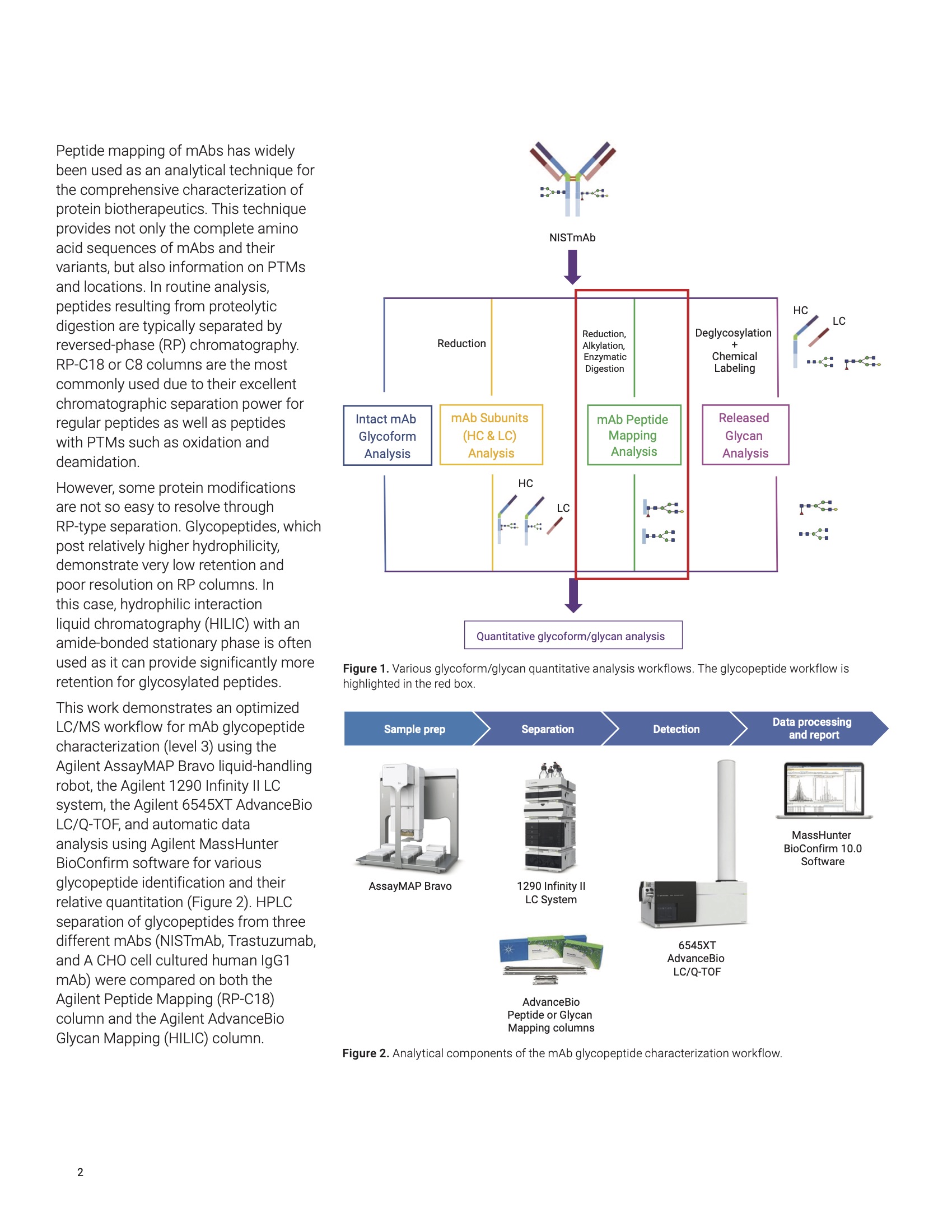
Monoclonal antibodies (mAbs) and their derivatives represent a very complex but important class of biopharmaceutical molecules with a wide range of applications. As mAbs are heterogeneous molecules by nature, comprehensive analytical characterization is required. The full range of biotherapeutics characterization usually includes confirmation of the protein sequences, protein post-translational modification (PTM) locations, and their relative quantitative information. Protein glycosylation is one of the major PTMs of an mAb, and is involved in many biological regulatory processes as well as therapeutic efficacy and immunogenicity1 . Therefore, it is important to understand the various glycans’ distribution and composition for pharmaceutical bioprocessing









 Tiếng Việt
Tiếng Việt
