Dissolution Workstation Software
Agilent Dissolution Workstation Software offers data integrity and automation to analysts using USP dissolution apparatus. Dissolution Workstation Software helps laboratory managers achieve secure and controlled access to methods and dissolution apparatus.
Specific access can be granted to different laboratory analysts with Dissolution Workstation Software. All user changes are tracked and electronically recorded. Secure tracking of what was done, when, and how, with all changes, facilitates 21 CFR Part 11 compliance for the regulated laboratory environment.
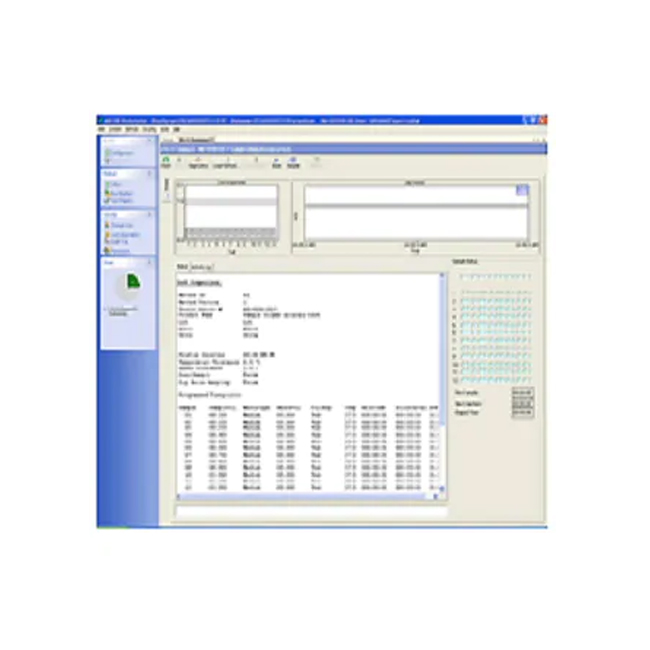
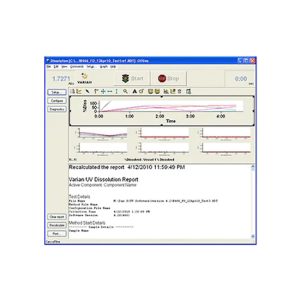
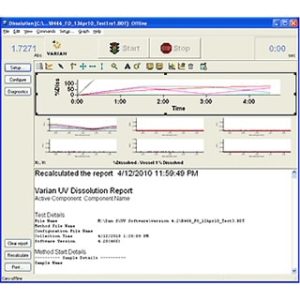
Dissolution Workstation Software is ideal for busy laboratory managers responsible for testing drug dosage forms like tablets, gels, capsules, and long-acting injectables. All method parameters, instrument parameters, plus accessory information and test data are captured and recorded.
This Dissolution Software lets you build, edit, search, retrieve, and archive all dissolution apparatus methods and dissolution accessories from a single interface. Laboratories testing generic drugs and branded pharmaceutical products use Dissolution Workstation Software for both efficient operation and data integrity.


 Tiếng Việt
Tiếng Việt