TRS100 Quantitative Pharmaceutical Analysis System
The TRS100 enables fast, easy-to-use whole tablet or capsule content uniformity and polymorph screening for pharmaceutical finished-product testing and formulation development. Easier to implement than other spectroscopic methods, Agilent’s transmission Raman spectroscopy (TRS) technology allows simple method development and deployment for quantitative analysis in quality control applications.
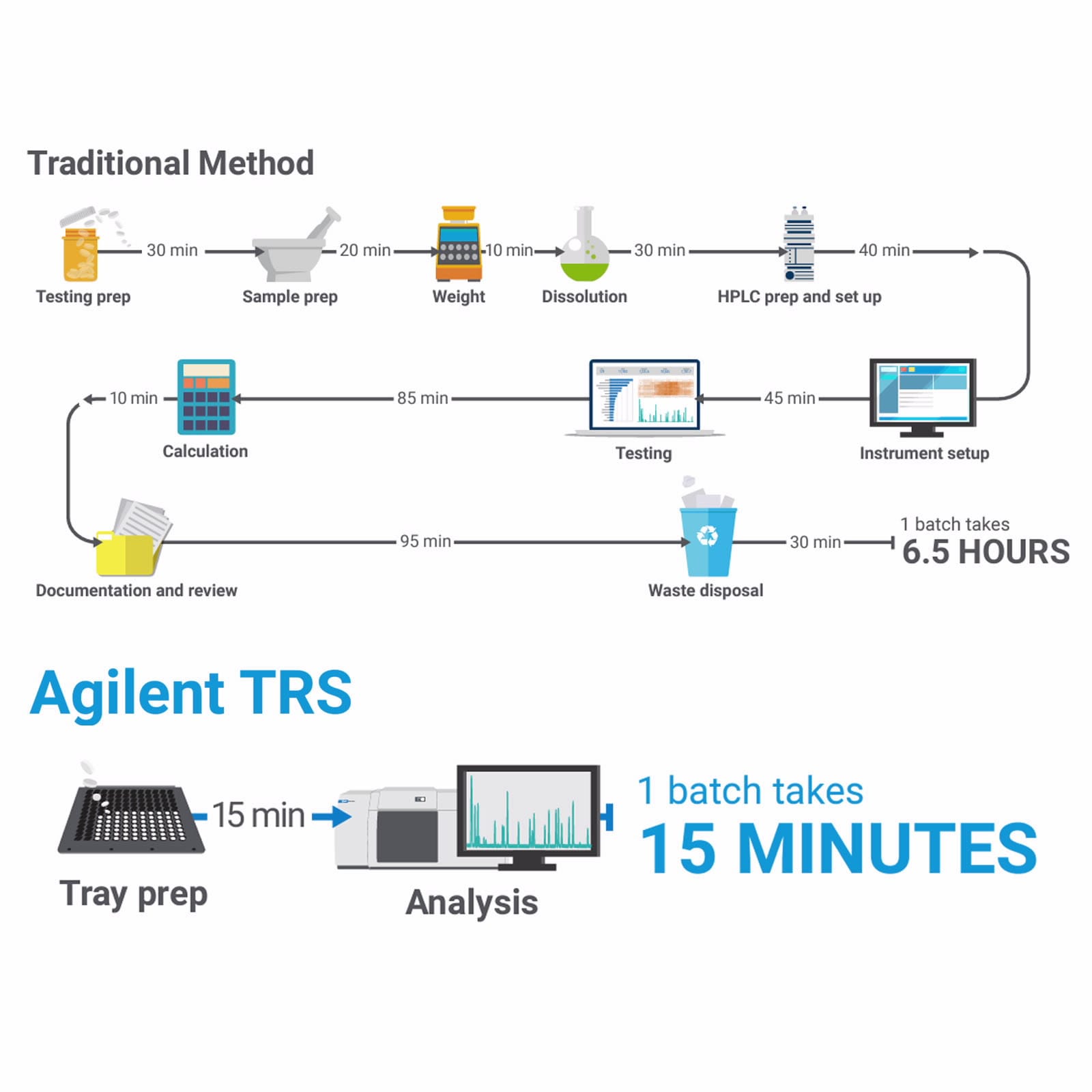
A TRS100 system can reduce content uniformity, assay, and ID tests to minutes per batch, saving significant cost and speeding up your quality control workflow. Sample preparation and consumables are not needed, meaning skilled analytical resources are not required. Content uniformity testing methods using transmission Raman have been approved by regulators using ICH and regulatory authority-acceptable protocols.


 Tiếng Việt
Tiếng Việt