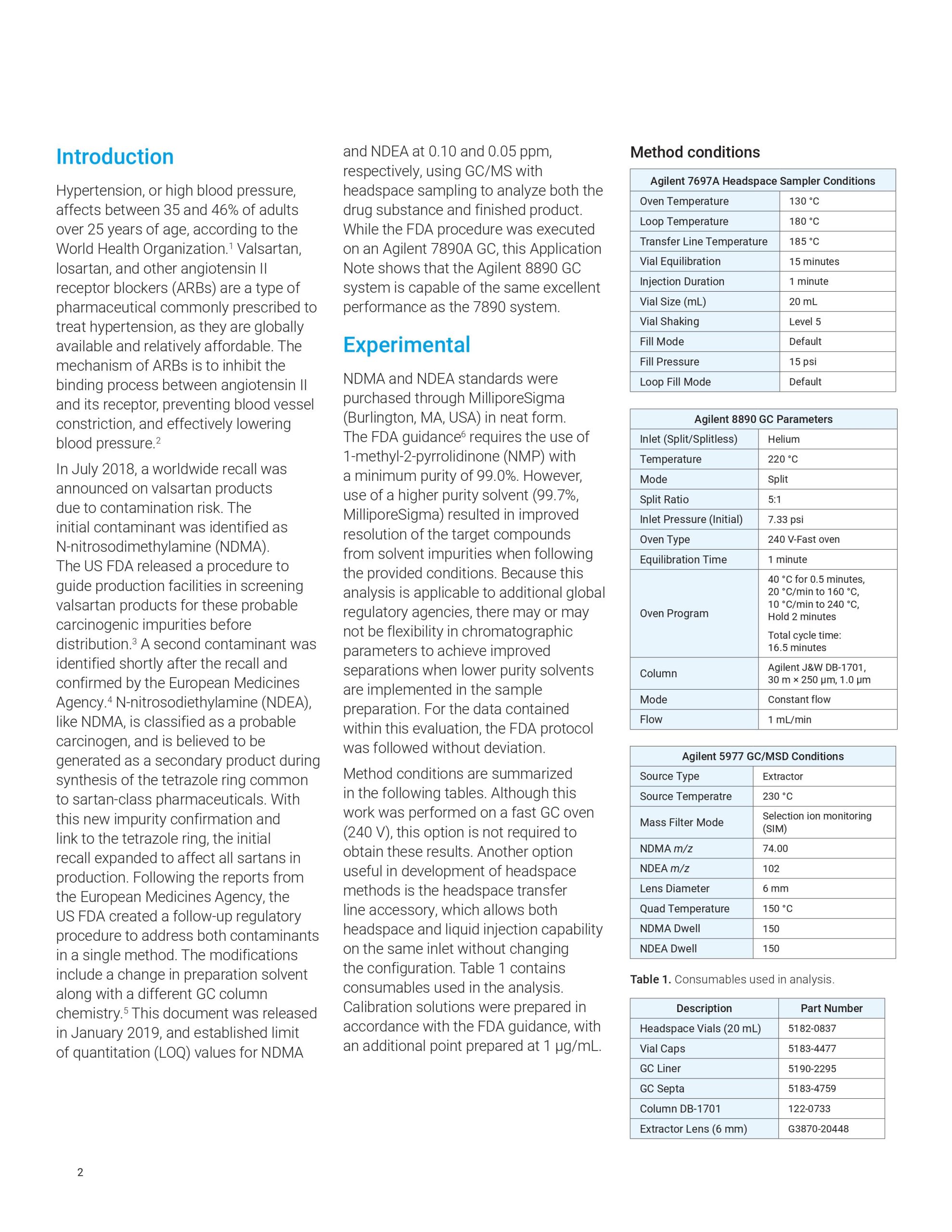
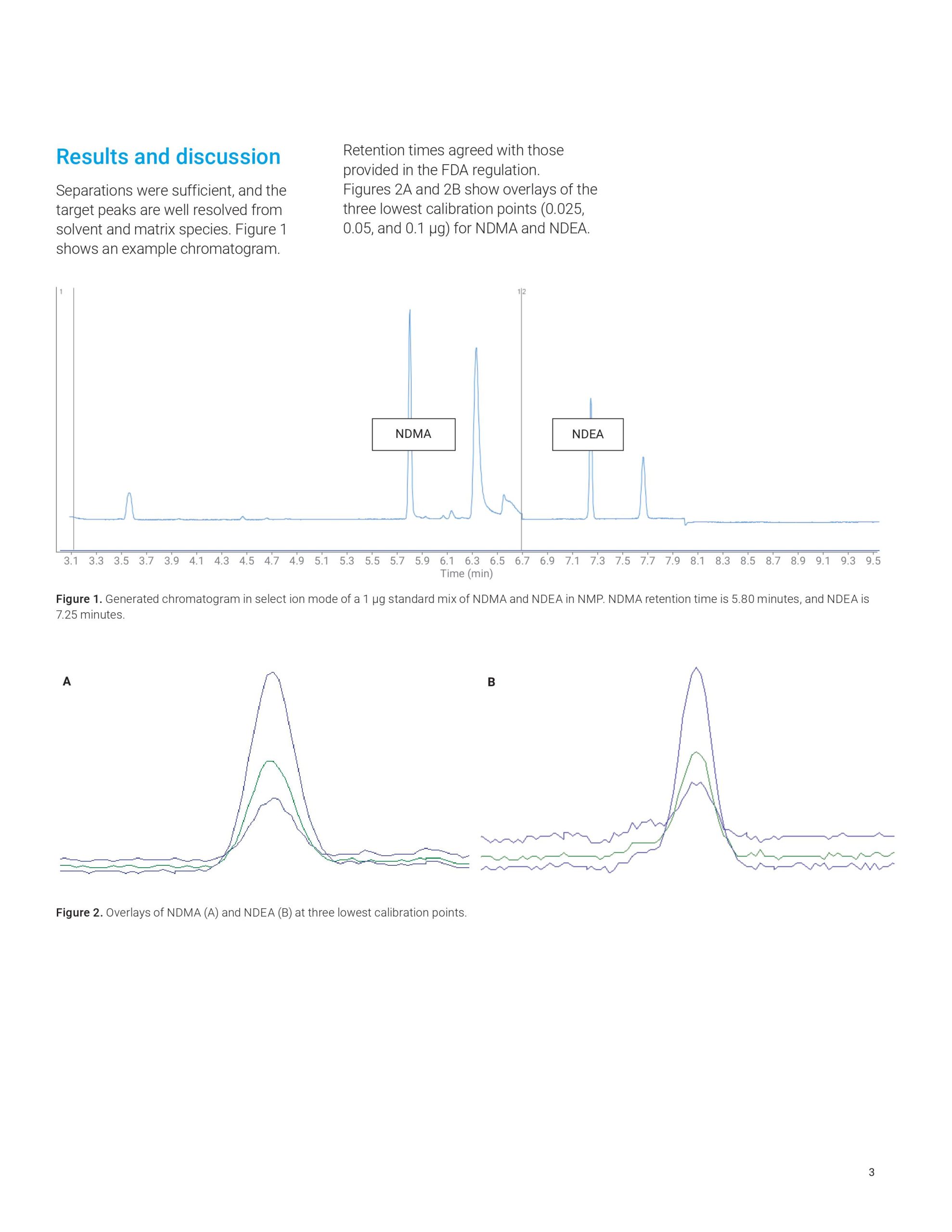
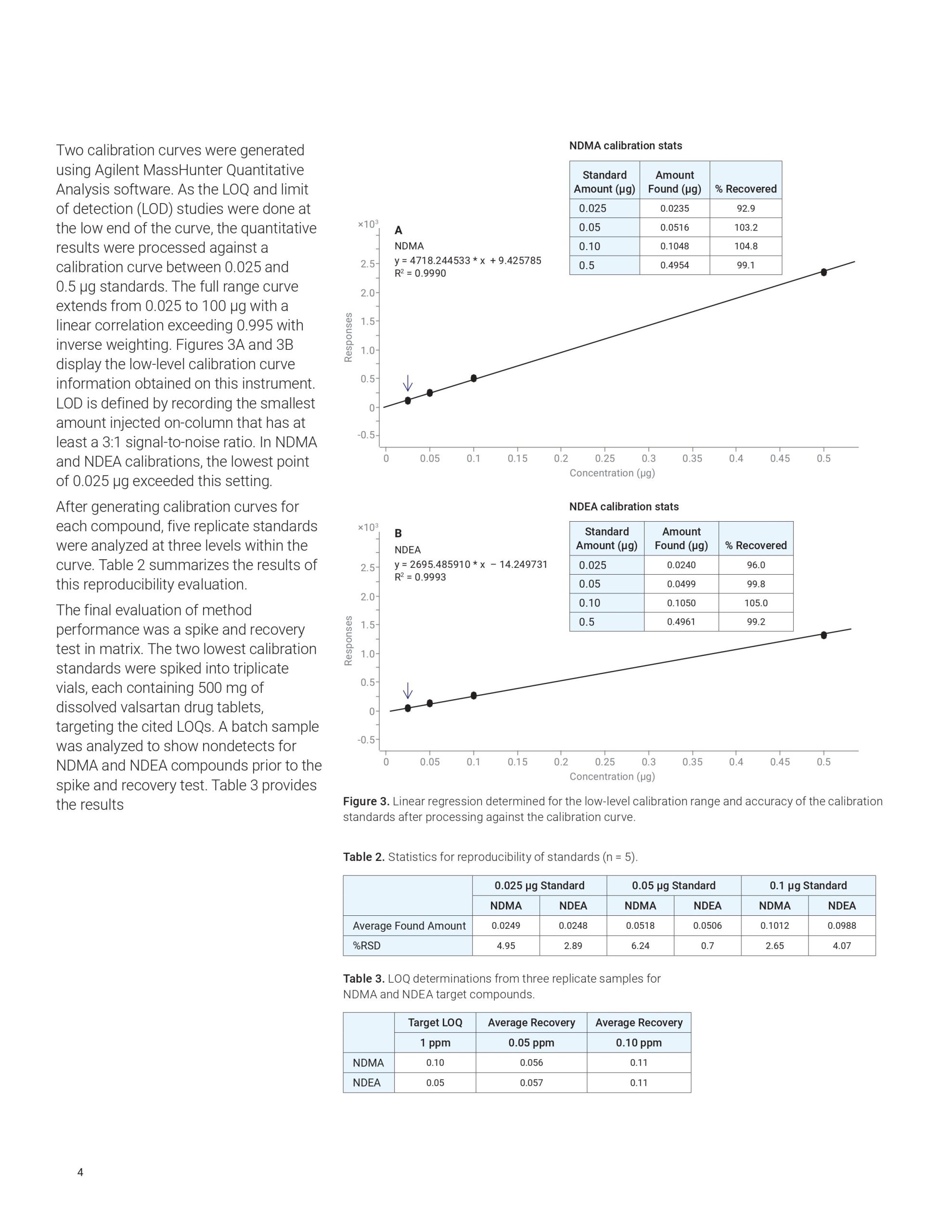
This Application Note describes the use of the Agilent 8890 GC to analyze impurities in sartan products according to a United States Food and Drug Administration (US FDA) method. The workflow also features an Agilent 7697A headspace sampler and a 5977 GC/MSD. Results corresponded to expected detection values.





 Tiếng Việt
Tiếng Việt
