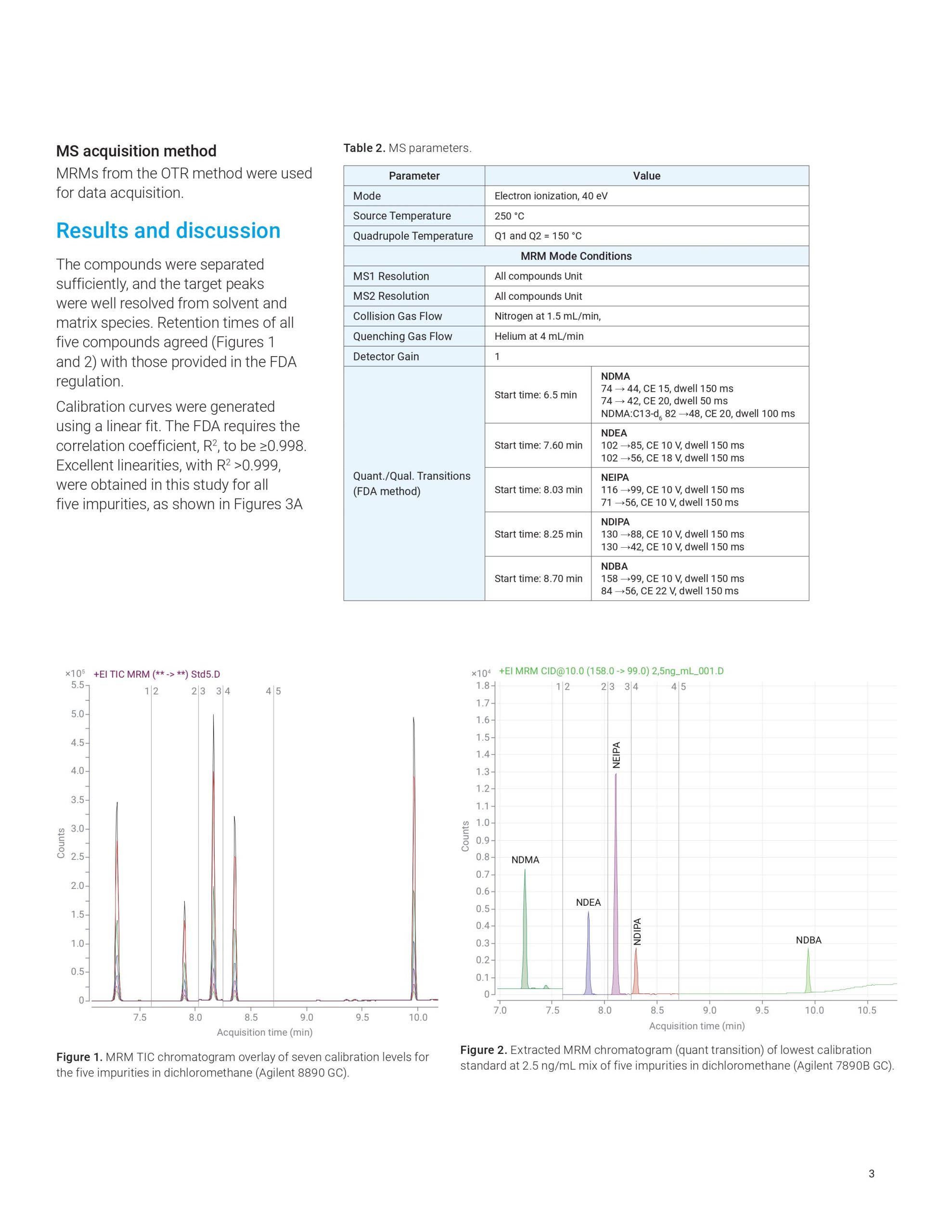
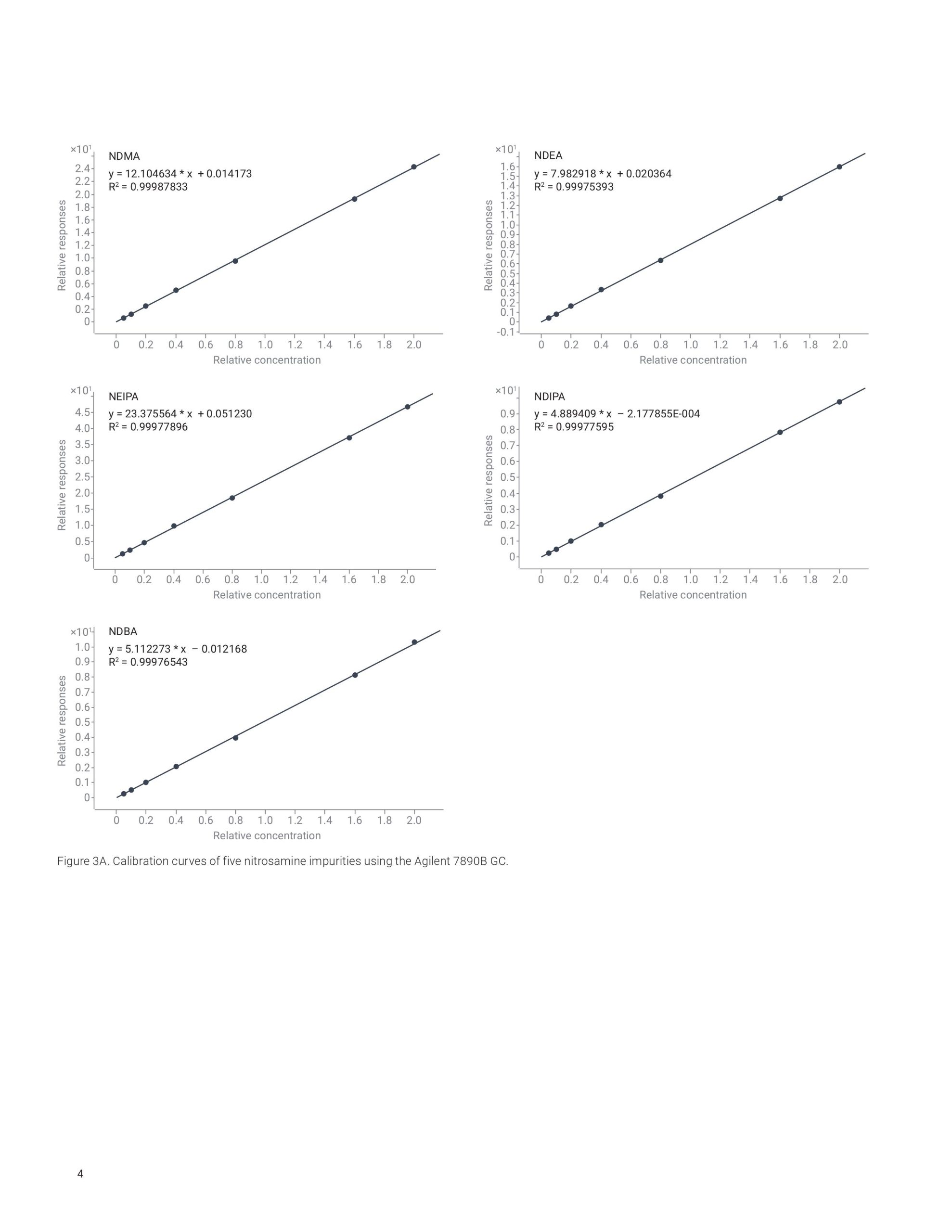
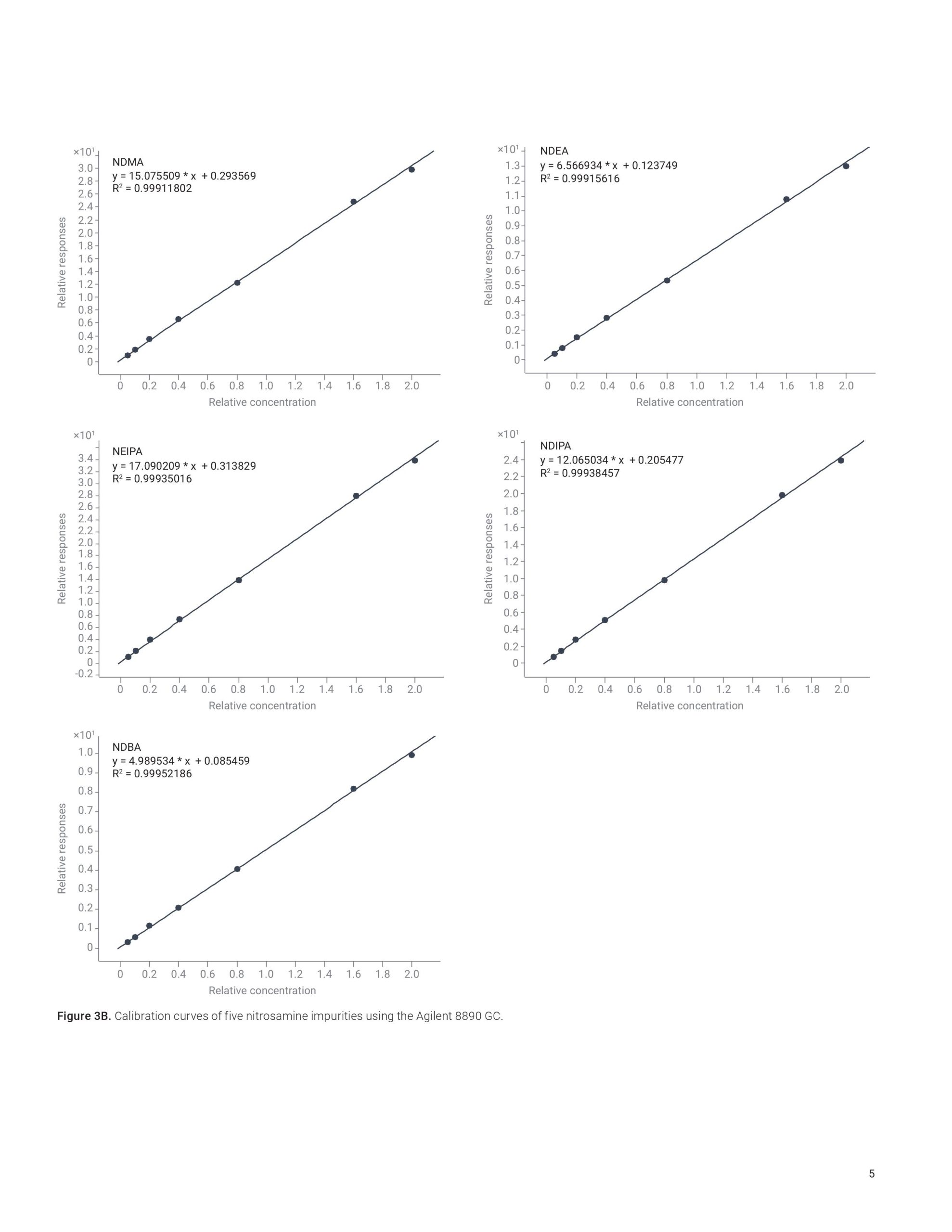
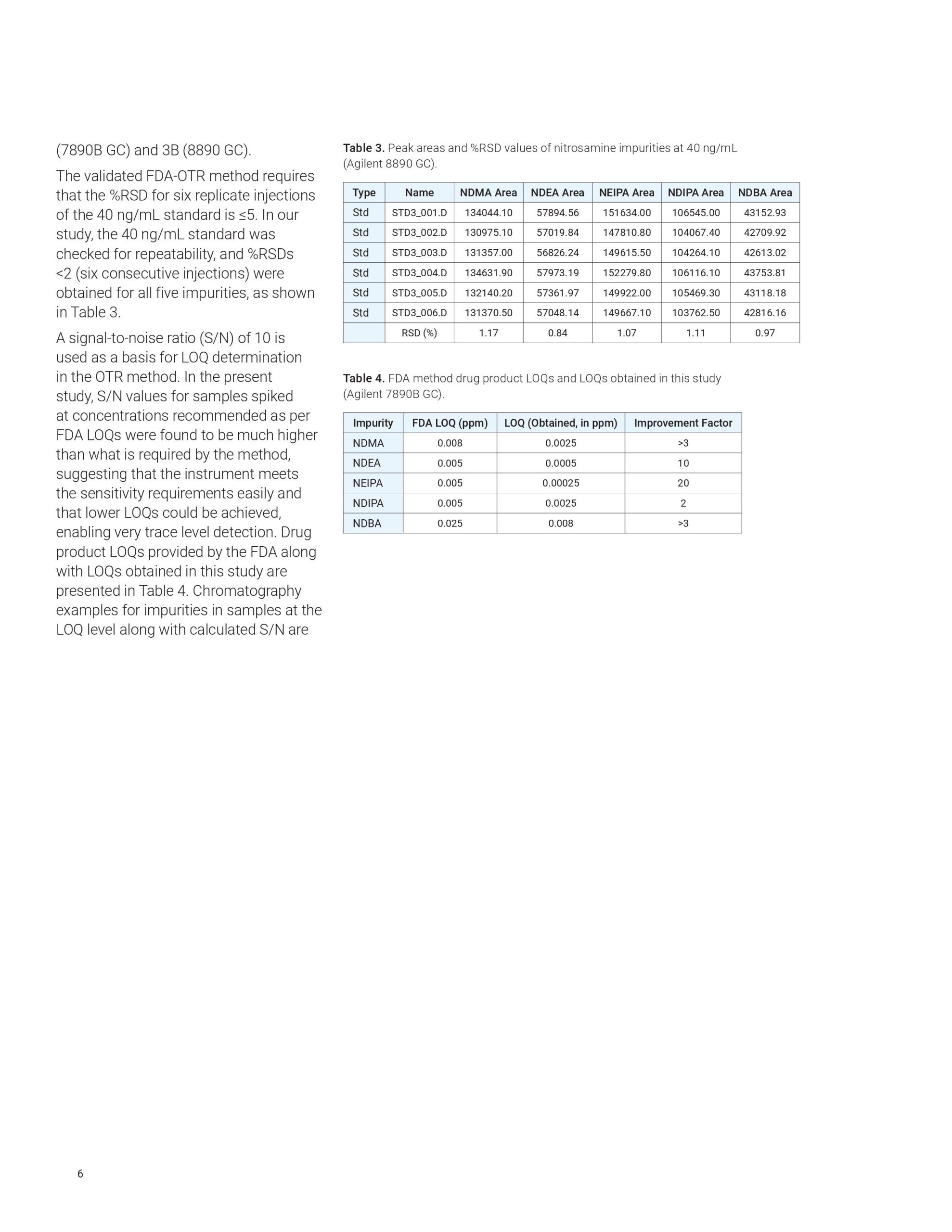
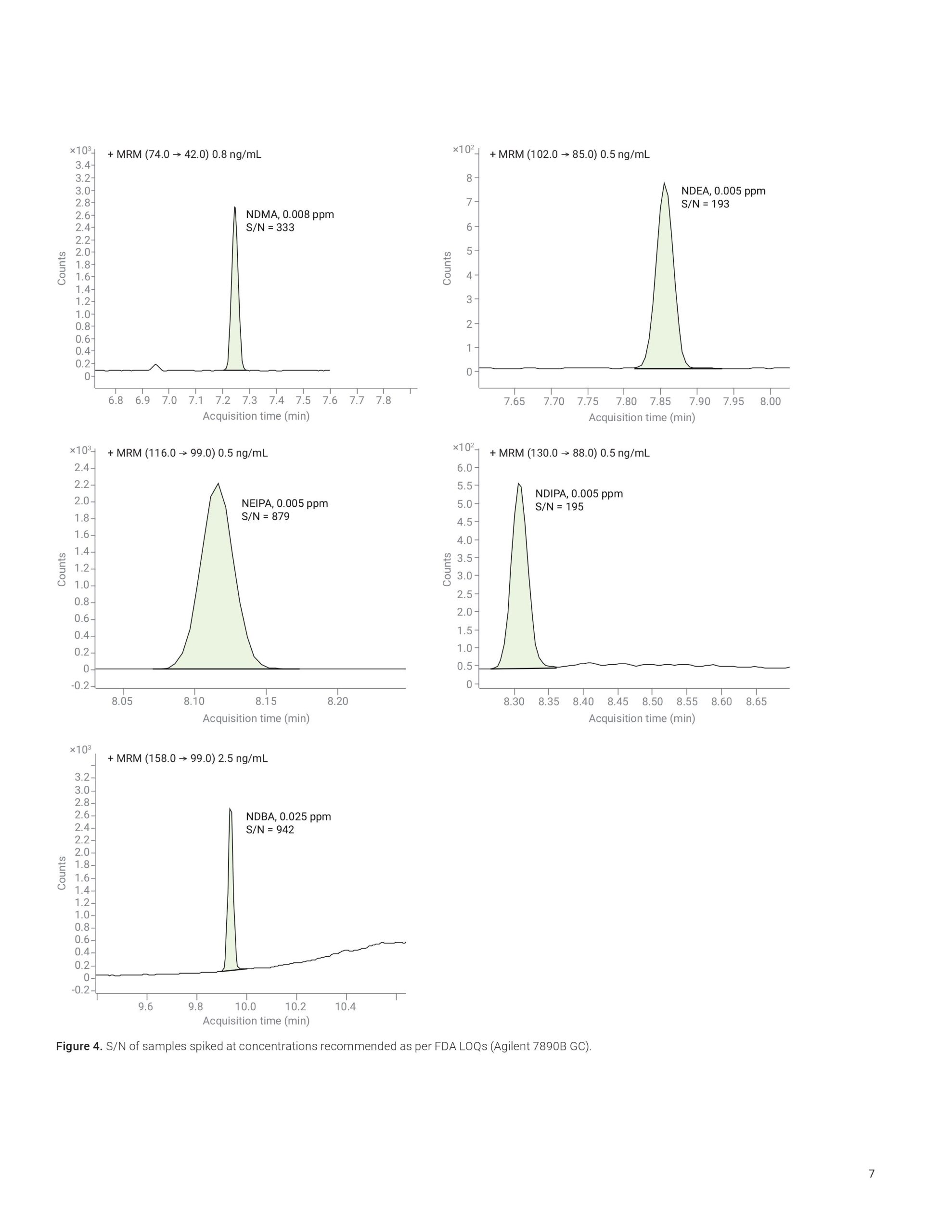
This application note highlights a comprehensive solution for the determination and estimation of five nitrosamine impurities (NDMA, NDEA, NEIPA, NDIPA, and NDBA) in sartan drug products and drug substances at trace levels using an Agilent 7890B or 8890 GC coupled to an Agilent 7010B triple quadrupole GC/MS system. The 7010B triple quadrupole GC/MS is equipped with a high-efficiency source (HES) that offers excellent sensitivity, repeatability, and precision while outperforming regulatory limits. The method allows for LOQs that are 2 to 20 times lower than what is required by current regulations.




 English
English
